 molecular structures. (Sometimes the symbols
molecular structures. (Sometimes the symbols  [or +·, "plus dot"] and
[or +·, "plus dot"] and  [or -·, "minus dot"] are used to
indicate radical [odd-electron] ions. This can be useful in understanding ion fragmentation patterns.)
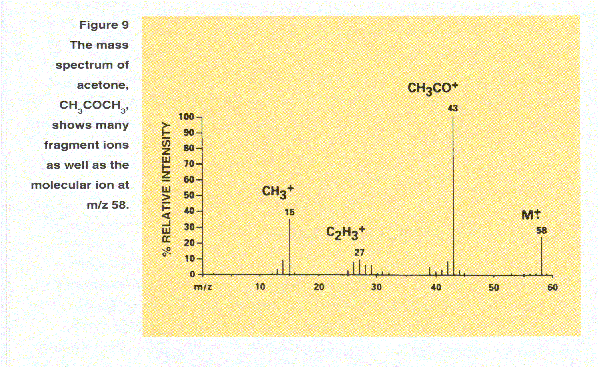
For example, the loss of 15 Da from the molecular ion of acetone to give an ion at m/z 43 indicates the presence
of a methyl group(CH3) in the original molecule. A subsequent loss of 28 Da to give an ion at m/z 15 suggests
the presence of CO. By rationalizing such losses and drawing reasonable structures for the resulting ions, the
structures of the original compounds can often be deduced. Some commonly observed lossed are 18 Da for water, H2O; 17 DA
for ammonia, NH3; and 77 Da for the phenyl group, C6H5.
[or -·, "minus dot"] are used to
indicate radical [odd-electron] ions. This can be useful in understanding ion fragmentation patterns.)
For example, the loss of 15 Da from the molecular ion of acetone to give an ion at m/z 43 indicates the presence
of a methyl group(CH3) in the original molecule. A subsequent loss of 28 Da to give an ion at m/z 15 suggests
the presence of CO. By rationalizing such losses and drawing reasonable structures for the resulting ions, the
structures of the original compounds can often be deduced. Some commonly observed lossed are 18 Da for water, H2O; 17 DA
for ammonia, NH3; and 77 Da for the phenyl group, C6H5.
Another aid in determining molecular composition is exact mass measurement. Every isotope of every element (except carbon which is assigned exactly 12.00000 Da) has a unique, non-integer mass. Exact mass measurement thus allows determination of chemical composition. As illustrated in Figure 10, with high resolution it is possible to distinguish between
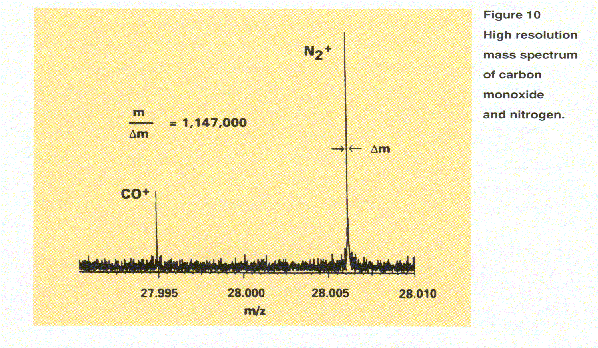 carbon monoxide (CO, m/z 27.995) and nitrogen (N2, m/z 28.006) by exact mass measurement. The spectrum shown in Figure 10
was recorded using an ultra-high resolution FT-ICR instrument. Notice that, unlike the simple histogram depictions of spectra
in Figures 2 and 9, this spectrum is shown as a plot of the acquired data.
carbon monoxide (CO, m/z 27.995) and nitrogen (N2, m/z 28.006) by exact mass measurement. The spectrum shown in Figure 10
was recorded using an ultra-high resolution FT-ICR instrument. Notice that, unlike the simple histogram depictions of spectra
in Figures 2 and 9, this spectrum is shown as a plot of the acquired data.
.gif)
