 energy or "soft" ionization techniques have been developed based on chemical
and desorption ionization.
energy or "soft" ionization techniques have been developed based on chemical
and desorption ionization.
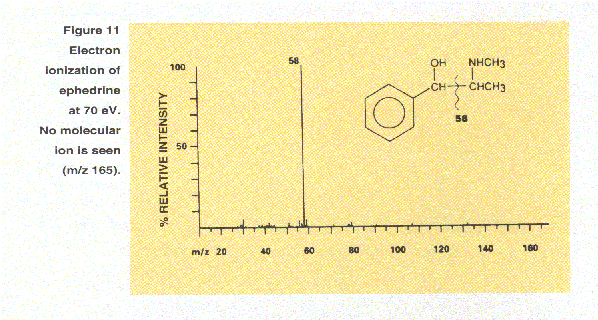
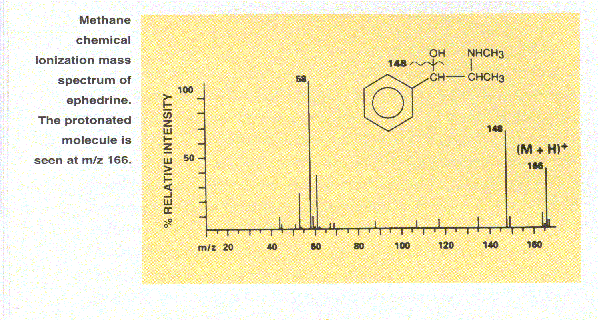
In contrast to electron ionization, most applications of chemical ionization (CI) produce ions by the relatively gentle process of proton transfer. The sample molecules are exposed to a large excess of ionized reagent gas. Transfer of a proton to a sample molecule M, from an ionized reagent gas such as methane in the form of CH5+, yields the [M+H]+ positive ion. For example, the mass spectrum of ephedrine (top spectrum of Figure 11) shows no molecular ion at m/z 165 under electron ionization conditions. However, under positive CI conditions (bottom spectrum of Figure 11), the protonated molecule at m/z 166 and the important fragement ion corresponding to the loss of water (18 Daltons) have significant intensity. In both spectra an intense ion is seen at m/z 58 but note that the fragmentation patterns of protonated molecules, [M+H]+, are not necessarily the same as the fragmentation patterns of molecular ions, M+.
Negative ions can also be produced under chemical ionization conditions. Transfer of a proton from M to other types of reagent gas or ions can leave [M-H]-, a negatively charged sample ion. Addition of an electron to M, a process facilitated by collisionally decreasing the energy of electrons generated in the source, can yield an intense M- ion. Such ions, often the only ion generated, can be used to detect species by mass spectrometry with great sensitivity.
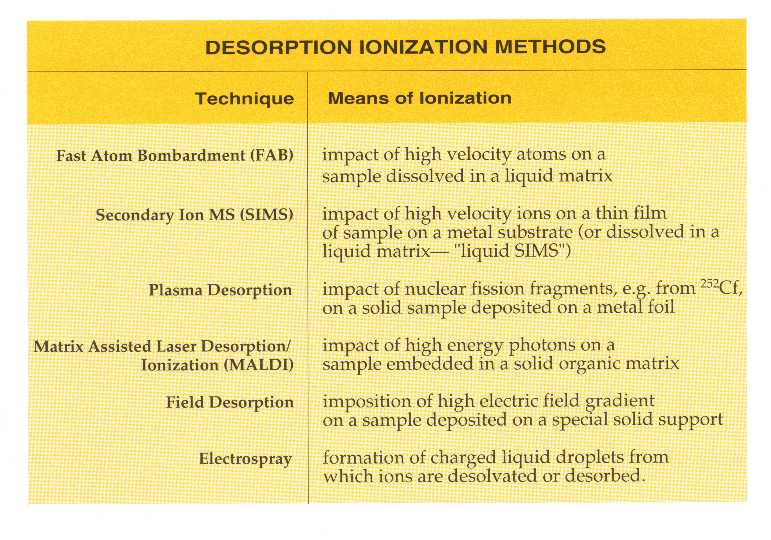
Desorption ionization is a term used by mass spectrometrists to decribe the process by which a molecule is both evaporated from a surface and ionized although the exact mechanism may not be understood. For the first four desorption ionization methods listed below, samples are desorbed and ionized by an impact process that involves bombardment of the sample with high velocity atoms, ions, fission fragments, or photons of relatively high energy. The impact deposits energy into the sample, either directly or via the matrix, and leads to both sample molecule transfer into the gas phase and ionization.
In field desorption, the sample is coated as a thin film onto a special filament placed within a very high intensity electric field. In this environment, ions created by field-induced removal of an electron from the molecule are extracted into the mass spectrometer.
.gif)
