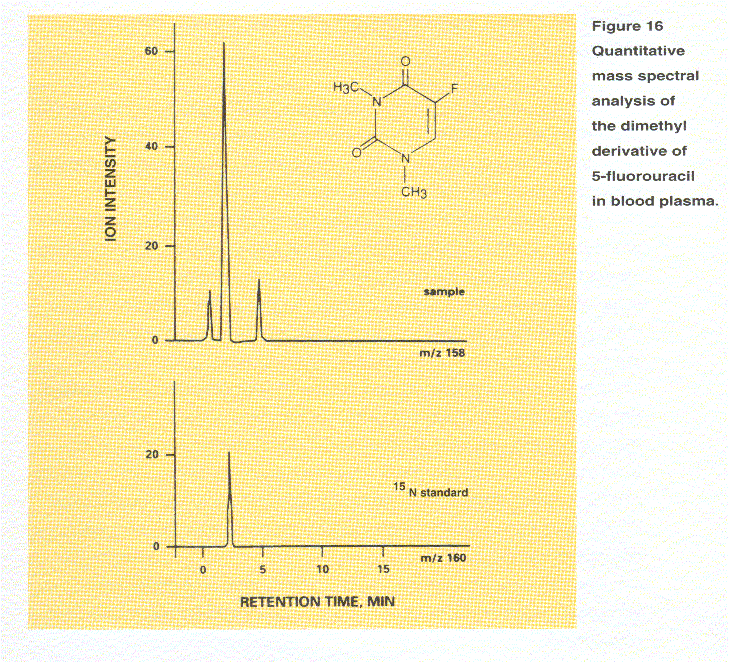
 A quantitative mass spectral analysis of the dimethyl
derivative of 5-fluorouracil in blood plasma is illustrated in Figure 16.
In this analysis, the response from the substance of interest is measured
relative to that from an internal standard added to the sample. The top trace
shows the SIM profile of the molecular ion at m/z 158. The compound of interest
produces the center peak. Two minor peaks arise from other sample components
that also produce ions at m/z 158. The lower trace is the SIM profile of the
same molecule having 15N substituted for the normal 14N at both nitrogen
positions. Since both nitrogens are substituted, the signal from its molecular
ion is observed at m/z 160, 2 Da higher. Although both the sample and standard
have the same retention time, they are detected separately by virtue of their
different masses. Standards may be a closely related substance or may be
chemically identical
but synthesized by substituting a isotope of one of the
elements as in this example.
A quantitative mass spectral analysis of the dimethyl
derivative of 5-fluorouracil in blood plasma is illustrated in Figure 16.
In this analysis, the response from the substance of interest is measured
relative to that from an internal standard added to the sample. The top trace
shows the SIM profile of the molecular ion at m/z 158. The compound of interest
produces the center peak. Two minor peaks arise from other sample components
that also produce ions at m/z 158. The lower trace is the SIM profile of the
same molecule having 15N substituted for the normal 14N at both nitrogen
positions. Since both nitrogens are substituted, the signal from its molecular
ion is observed at m/z 160, 2 Da higher. Although both the sample and standard
have the same retention time, they are detected separately by virtue of their
different masses. Standards may be a closely related substance or may be
chemically identical
but synthesized by substituting a isotope of one of the
elements as in this example.
Home
.gif)
