When vaporized, the solvent from a liquid chromatograph represents a volume of 100-1000 times greater than that of a carries gas used in gas chromatography. Interfaces developed commercially over the last decade have solved the problem of eliminating this gas load by using combinations of heating and pumping, sometimes with the assistance of a drying gas stream. The inlets for higher flow rates (as in analytical LC) employed in LC/MS systems in routine use today include atmospheric pressure chemical ionization (APCI), electrospray, thermospray and particle beam interfaces.
In atmospheric pressure chemical ionization inerfaces, the solution from the LC passes through a heated nebulizer into the APCI source. In electrospray ionization interfaces at higher solvent flow rates, heat and drying gas are usually needed to increase the rate of droplet evaporation as the sample solution is sprayed from a needle held at high voltage. (A unique feature of both the APCI and electrospray interfaces is that the sample enters the vacuum region of the mass spectrometer already in the form of ions.) In thermospray, heat is applied to evaporate the solvent as the sample solution is sprayed into a moderate vacuum. In the particle beam interface, lighter solvent molecules are evaporated by the application of heat and a momentum separator retains the heavier sample-containing particles for passage into the mass spectrometer. For low solvent flow rates (as in microcolumn LC), direct introduction can be used with chemical ionization or electrospray ionization (flow rates of a few microliters per minute or flow rates of several hundred microliters per minute using mechanical nebulization of the liquid stream).
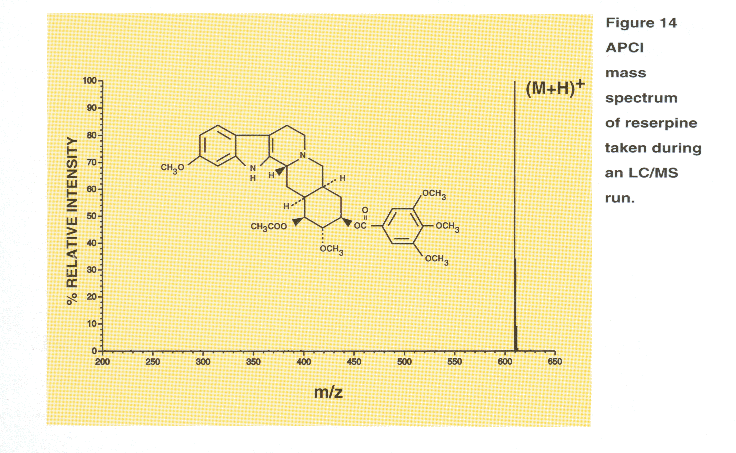 Shown in Figure 14 is an APCI mass spectrum of reserpine. The
potonated molecule appears at m/z 609. This spectrum was taken during a 400
uL/minute gradient LC run using an acetonitrile/water/0.1% trifluoroacetic acid
solvent system.
Shown in Figure 14 is an APCI mass spectrum of reserpine. The
potonated molecule appears at m/z 609. This spectrum was taken during a 400
uL/minute gradient LC run using an acetonitrile/water/0.1% trifluoroacetic acid
solvent system.
For GC/MS, LC/MS or other combinations, the data consists of a series of mass spectra that are acquired sequentially in time. To generate this information, the mass spectrometer scans the mass range (e.g., m/z 30-500) repetitively during the chromatographic run.
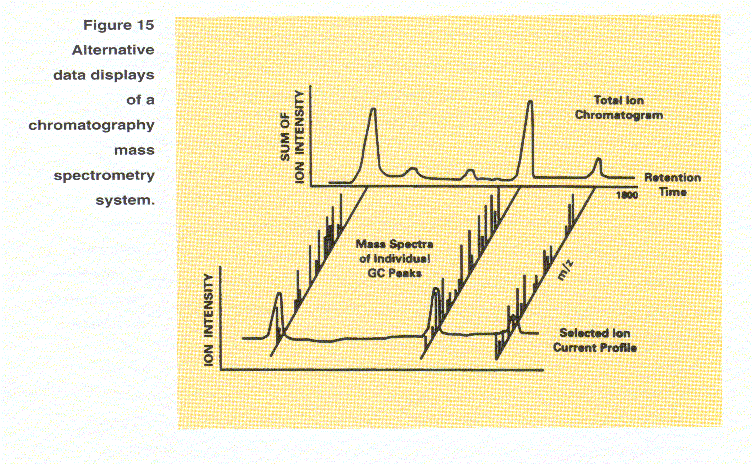 If a scan is taken every second
and the run is 30 minutes long, 1800 spectra are recorded. This information may be
displayed in several ways as shown in Figure 15. First the intensities of all the
ions in each spectrum can be summed, and this sum plotted as a function of
chromatographic retention time to give a total ion chromatogram (TIC) whose
appearance is similar to the output of a conventional chromatographic detector.
Second, as shown in the diagonal display in Figure 15, any of the spectra can be
diplayed. Each peak in the TIC represents an eluding compound that can be
identified by interpretation of the mass spectra recorded for the peak. Finally,
as shown in the lower part of Figure 15, the intensity at a single mass-to-charge
ratio over the course of a chromatographic run can be displayed to yield a selected
ion current profile or mass chromatogram. This technique can be used to find
components of interest in a complex mixture without having to examine each
individual mass spectrum.
If a scan is taken every second
and the run is 30 minutes long, 1800 spectra are recorded. This information may be
displayed in several ways as shown in Figure 15. First the intensities of all the
ions in each spectrum can be summed, and this sum plotted as a function of
chromatographic retention time to give a total ion chromatogram (TIC) whose
appearance is similar to the output of a conventional chromatographic detector.
Second, as shown in the diagonal display in Figure 15, any of the spectra can be
diplayed. Each peak in the TIC represents an eluding compound that can be
identified by interpretation of the mass spectra recorded for the peak. Finally,
as shown in the lower part of Figure 15, the intensity at a single mass-to-charge
ratio over the course of a chromatographic run can be displayed to yield a selected
ion current profile or mass chromatogram. This technique can be used to find
components of interest in a complex mixture without having to examine each
individual mass spectrum.
Home
.gif)
