Magnetic sectors bend the trajectories of ions into circular paths of radii that depend on the momentum-to-charge ratios of the ions. Ions of larger m/z follow larger radius paths than ions of smaller m/z values so ions of differing m/z values are dispersed in space. By changing the ion trajectories through variations of the magnetic field strength, ions of different nominal mass-to-charge ratios can be focused on a detector.
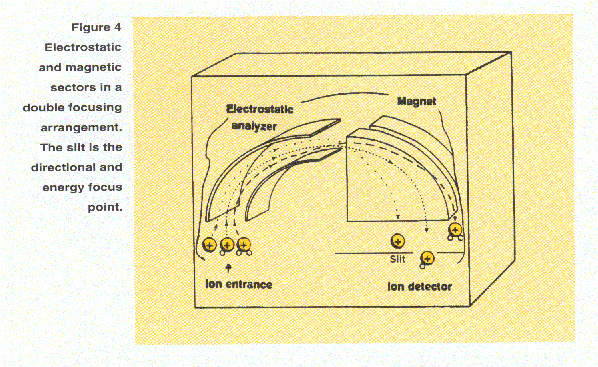
Double focusing mass spectrometers use a combination of magnetic and electrical fields to focus and sort ions. A common configuration for a sector instrument is the geometry shown in Figure 4, in which a magnetic "sector" follows an electric "sector". The slit acts as a filter to select for a specific m/z value. The electric sector focuses the ions with respect to differences in kinetic energy that they may have as they exit the source region. "Double focusing," this combination of "angular" or "directional" focusing and energy focusing, provide mass resolution high enough to separate ions of the same nominal mass but different chemical formulae, such as C2H4, N2 and CO at m/z 28. The so called "exact masses", more properly "high precision masses", of C2H4, N2 and CO are 28.0313, 28.0061, and 27.9949 Daltons, respectively2.
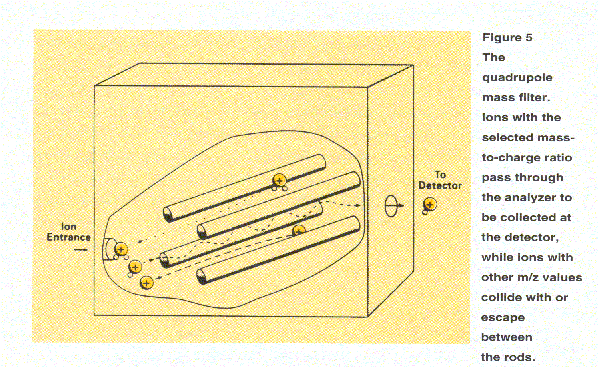
Another type of mass analyzer, called a quadrupole mass filter, consists of four parallel poles or rods. In this device (Figure 5), mass sorting depends on ion motion resulting from simultaneously applied constant (dc) and radio frequency electric (rf) electric fields. Scanning is accomplished by systematically changing the field strengths, thereby changing the m/z value that is transmitted through the analyzer. Quadrupole mass spectrometers provide lower resolution than double focusing instruments but tend to be more easily interfaced to various inlet systems and to be less costly.
The quadrupole ion trap mass spectrometer (Figure 6)
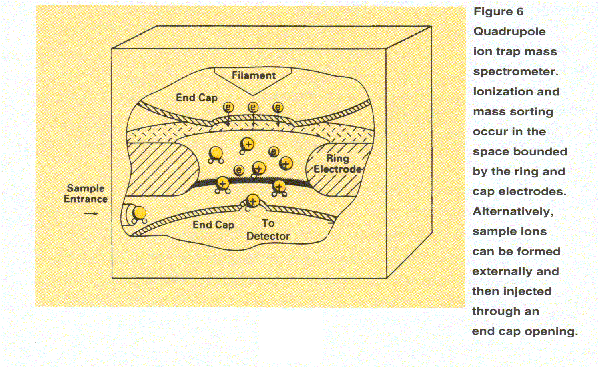 operates on a principle similar to
a quadrupole mass filter. However, it does not operate as a filter.
Rather, the ion trap stores ions for subsequent experiments and analysis.
It uses fields
generated by rf (and sometimes dc) voltages applied to electrodes arranged
in a sandwich geometry: a ring electrode in the middle
with cap electrodes on each end. Within a selected range of mass-to-charge ratios
determined by the applied voltages, the device traps ions in the space bounded
by the electrodes. Typically, a mass spectrum is produced by scanning the applied
rf voltages to eject ions sequentially of increasing mass-to-charge ratio through
an end cap opening for detection.
operates on a principle similar to
a quadrupole mass filter. However, it does not operate as a filter.
Rather, the ion trap stores ions for subsequent experiments and analysis.
It uses fields
generated by rf (and sometimes dc) voltages applied to electrodes arranged
in a sandwich geometry: a ring electrode in the middle
with cap electrodes on each end. Within a selected range of mass-to-charge ratios
determined by the applied voltages, the device traps ions in the space bounded
by the electrodes. Typically, a mass spectrum is produced by scanning the applied
rf voltages to eject ions sequentially of increasing mass-to-charge ratio through
an end cap opening for detection.
Two other analyzers now being used frequently are the Fourier transform ion cyclotron resonance (FT-ICR) spectrometer and the time-of-flight (TOF) mass spectrometer. The unique capabilities of each of these mass analyzers make them especially useful as mass spectrometry moves into new areas of application.
In an FT-ICR spectrometer (Figure 7)
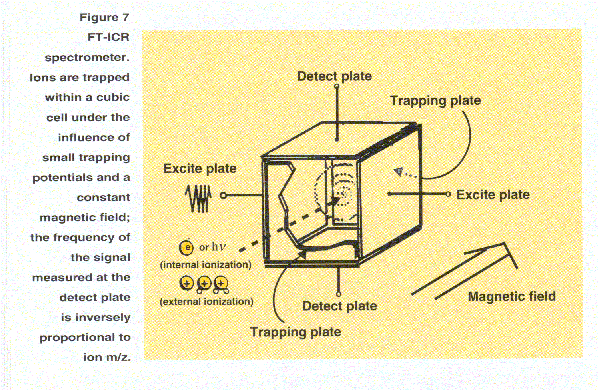 ions are trapped electrostatically
within a cubic cell in a constant magnetic field. A covalent orbital
("cyclotron") motion is induced by the application of a radio-frequency pulse between
the excite plates. The orbiting ions generate a faint signal
in the detect plates of the cell. The frequency of the signal from
each ion is equal to its orbital frequency, which in turn is inversely
related to its m/z value. The signal intensity of each frequency is
proportional to the number of ions having that m/z value. The signal is amplified
and all the frequency components are determined, yielding the mass spectrum.
If the pressure in the cell is very low, the
ion orbital motion can be maintained over many cycles and the frequency can be
measured with very high precision. The FT-ICR instrument can therefore
be used to generate very high resolution spectra.
ions are trapped electrostatically
within a cubic cell in a constant magnetic field. A covalent orbital
("cyclotron") motion is induced by the application of a radio-frequency pulse between
the excite plates. The orbiting ions generate a faint signal
in the detect plates of the cell. The frequency of the signal from
each ion is equal to its orbital frequency, which in turn is inversely
related to its m/z value. The signal intensity of each frequency is
proportional to the number of ions having that m/z value. The signal is amplified
and all the frequency components are determined, yielding the mass spectrum.
If the pressure in the cell is very low, the
ion orbital motion can be maintained over many cycles and the frequency can be
measured with very high precision. The FT-ICR instrument can therefore
be used to generate very high resolution spectra.
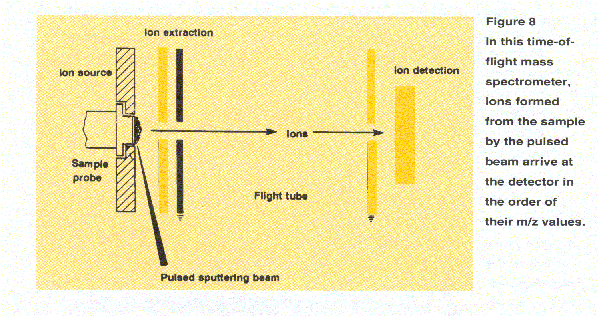
Time-of-flight mass analyzers (Figure 8) seperate ions by virtue of their different flight times over a known distance. A brief burst of ions is emitted from a source. These ions are accelerated so that ions of like charge have equal kinetic energy and then are directed into a flight tube. Since kinetic energy
 is equal to 1/2 mv2, where m is the
mass of the ion and v is the ion
velocity, the lower the ion's mass, the greater the velocity and shorter its
flight time. The travel time from the ion source through the flight tube to
the detector, measured in microseconds, can be transformed to the m/z value
through the relationships described above. Because all ion masses are
measured for each ion burst, TOF mass spectrometers offer high
sensitivity as well as rapid scanning. They can provide mass data
for very high-mass biomolecules.
is equal to 1/2 mv2, where m is the
mass of the ion and v is the ion
velocity, the lower the ion's mass, the greater the velocity and shorter its
flight time. The travel time from the ion source through the flight tube to
the detector, measured in microseconds, can be transformed to the m/z value
through the relationships described above. Because all ion masses are
measured for each ion burst, TOF mass spectrometers offer high
sensitivity as well as rapid scanning. They can provide mass data
for very high-mass biomolecules.
.gif)
