What are the characteristics of a mass spectrum?
A mass spectrum is a graph of ion intensity as a function of mass-to-charge ratio.
Mass spectra are often depicted as simple histograms as shown in Figure 2.
This record of ions and their intensities serve to establish the molecular weight and structure of the
compound being mass
analyzed. For example, Figure 2 shows a mass spectrum of the simple molecule carbon dioxide, CO2.
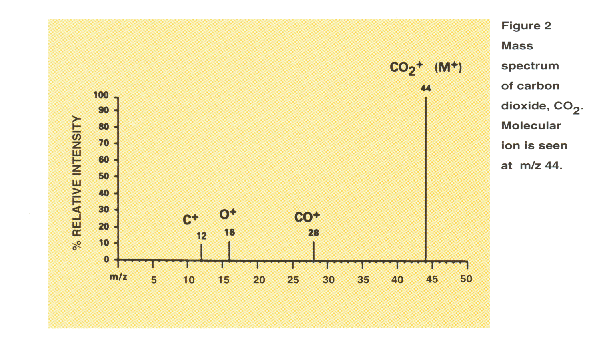
In this example, all the ions are positively charged. (It is possible
to generate and detect negative ions as well.) The ionized CO2 molecule (or molecular ion)
appears at m/z 441. Since the ionization process breaks up or fragments some of the CO2
molecules, a fraction of the ions appear in the spectrum at m/z values less than the m/z value that corresponds
to the molecular mass of CO2. Cleavage of a carbon-oxygen bond in the
molecular ion to produce ionized
carbon
monoxide or ionized atomic oxygen result in the fragment ions at m/z 28 and 16; loss of two neutral oxygen
atoms results in an additional fragment at m/z 12 for carbon. The molecular ion is designated
as M+ or CO2+ and the fragment ions are designated as CO+, O+
and C+.
1 The ion is singly charged and the "nominal ion mass" is 44 Da: carbon=12 and oxygen=16
(in calculating nominal ion mass, atomic masses are rounded to the nearest integer).
Home
.gif)


.gif)
